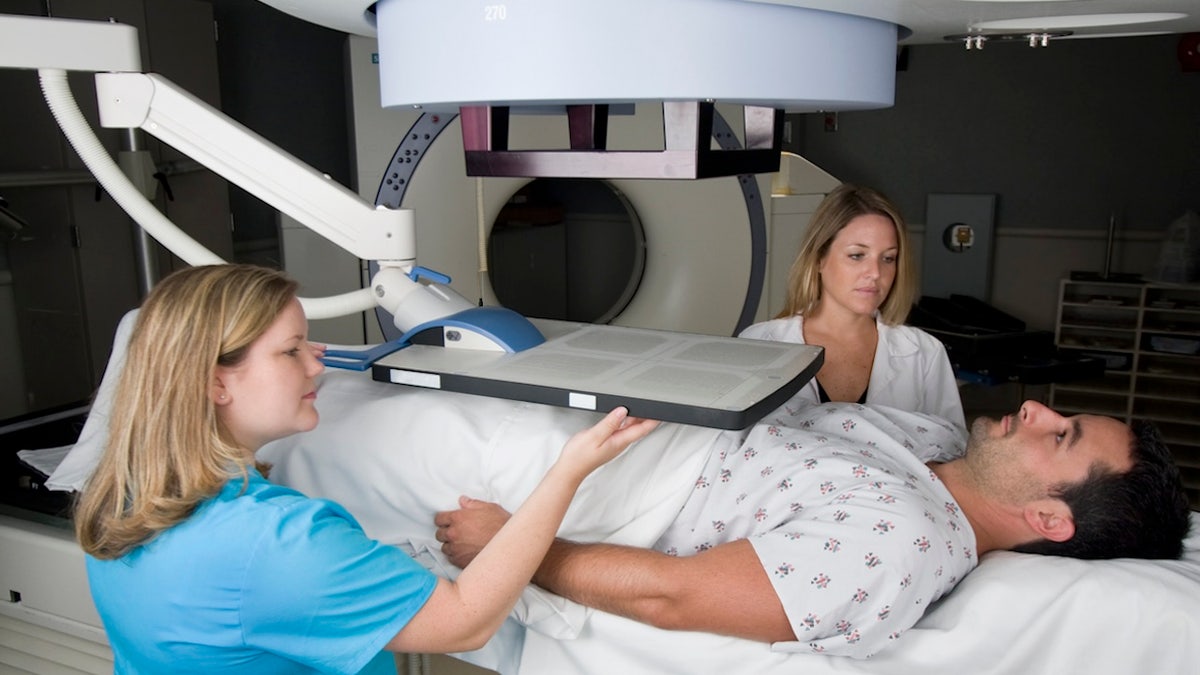
A recently expanded prostate cancer medication could bring new hope to patients with a common form of the disease.
Novartis, a pharmaceutical company based in Switzerland, announced on March 28 that the United States Food and Medicines Administration (FDA) has expanded the approval of pluvicto (Lutetium Lu 177 Vipotide Tetraxetan), a radioliganda therapy (RLT) directed before chemotherapy.
(RLT are a form of directed nuclear medicine that doctors use to treat multiple types of cancer, according to Novartis).
The risk of prostate cancer increases by 45% among men who share a worrying behavior
The drug is intended for patients with positive metastatic castration cancer for PSMA (MCRPC) who have received a round of androgen receiver inhibitors (ARPI), a class of medications used in the treatment of metastatic prostate cancer.

A recently expanded prostate cancer medication could bring new hope to patients with a common form of the disease. (Istock)
Pluvicto first obtained the approval of the FDA on March 23, 2022, but this new approval expanded triples the number of patients eligible to receive the medicine, according to a press release from Novartis.
The medicine is administered through an IV to the bloodstream, where it joins the cancer cells of prostate and prevents them from replicating or killed.
Prostate cancer cases increase in this US state. As doctors share a probable reason
“The previous indication for rainfall could really change our treatment paradigms for patients with MCRPC,” said the main author of the study Michael Morris, MD, head of the prostate cancer section at the Sloan Kettering Memorial Cancer Center in New York.
“It offers specific therapy that better delays the progression of the disease compared to a second ARPI. This approval is a significant step forward and must open the door to a therapy that has clear clinical advantages for the patient with MCRPC that has progressed in an ARPI and has not received chemotherapy.”
In clinical trials, rainfall “significantly reduced the risk of progression or death” in 59%.
This is a form of prostate cancer that has spread to other parts of the body and does not respond to standard hormonal therapy, according to WebMD.
It also has high levels of specific prostate membrane (PSMA), a protein produced by prostate cancer cells.
In clinical trials, rainfall “significantly reduced the risk of progression or death” in 59% in patients with MCRPC, Novartis reported.
IMAGE
“The expanded approval of the FDA of [lutetium Lu 177 vipivotide tetraxetan] It marks a transformative step in the treatment of MCRPC, underlining the growing impact of precision oncology “, Jorge A. García, MD, a genitourinary medical oncologist and president of the solid division of tumor oncology in university hospitals Seidman Cancer Center/Case Western Reerve University in Cleveland, Ohio, said in Clive.
Click here to get the Fox News application
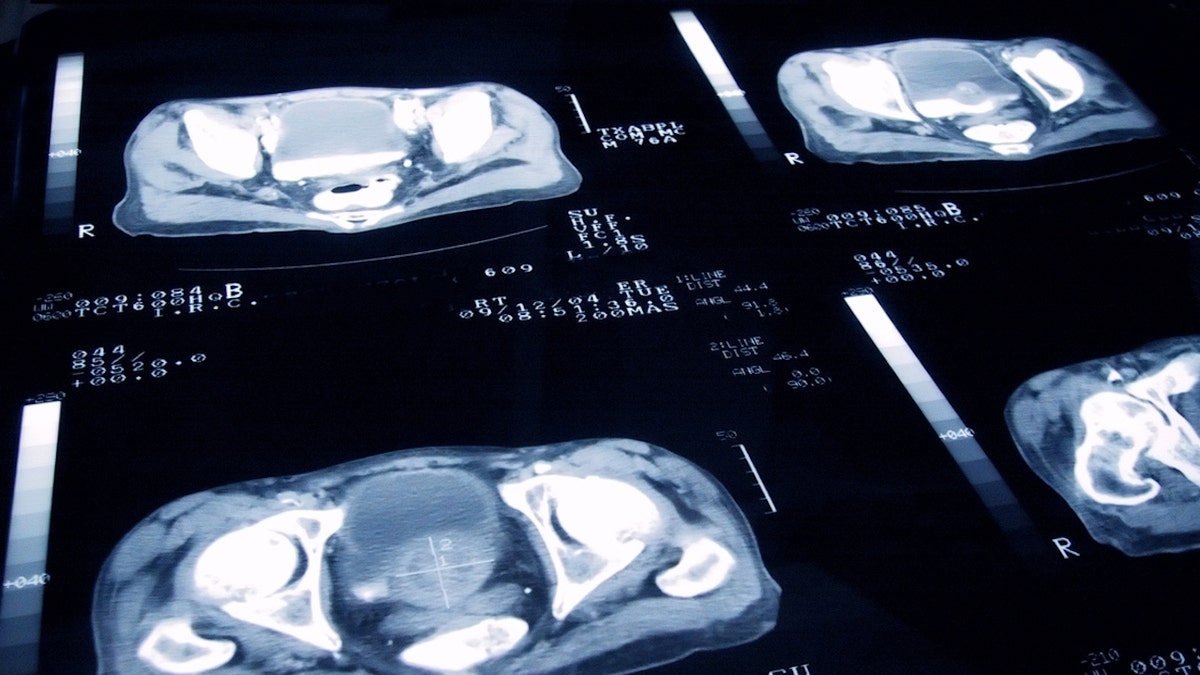
“By allowing access to this radioligand therapy directed before chemotherapy, we are not only expanding treatment options, but also redefining the standard of care for positive disease for PSMA.”
“By allowing access to this radioligand therapy directed before chemotherapy, we are not only expanding treatment options, but also redefining the standard of care for positive disease for PSMA,” said a researcher. (Istock)
Prostate cancer is the second main cause of death among men; MCRPC constitutes most deaths and 20% of all cases of metastatic prostate cancer.
Studies have shown that approximately 10% to 20% of prostate cancer patients develop MCRPC within five years after follow -up after initial therapy, and cases of metastatic patients have increased from 4% to 5% each year since 2011.
Click here to register in our health newsletter
Sixty percent of prostate cancers are diagnosed in men 65 years of age or older, according to the American cancer society. The risk of being diagnosed with metastatic prostate cancer occurs typically between 65 and 74.
The adverse rainfall effects included dry mouth (61%), fatigue (53%), nausea (32%) and constipation (22%), according to release.
Prostate cancer is the second main cause of death among men; MCRPC constitutes most deaths and 20% of all cases of metastatic prostate cancer. (Istock)
The patients who received the medication were able to proceed with chemotherapy after taking it.
Novartis undertakes to deliver rainfall to the almost 600 RLT treatment sites in the United States, the company said.
For more health articles, visit www.foxnews.com/health
Looking towards the future, Novartis said it plans to investigate the use of RLT for other types of advanced cancers, including breast cancers, colon, neuroendocrine, lungs and pancreatic.